Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Otobe, Haruyoshi; Kitatsuji, Yoshihiro; Yamamoto, Masahiro
ECS Transactions, 75(27), p.51 - 57, 2017/01
Times Cited Count:1 Percentile:38.46(Electrochemistry)We investigated the deposition of U(IV) following a valence change of U as electrodeposition using an electrochemical quartz crystal microbalance (EQCM). When measurements of the reduction of U(VI) in a weak acid solution were performed, deposits of U(IV) were observed on the electrode surface. From deposition rates, pH dependence of them, and oxidation potentials of deposits, we proposed the following deposition mechanism. The deposition is divided into the three phases; First, in the induction phase, U(IV) produced by the disproportionation forms U(IV) hydroxide nucleus. Next, in the growth phase, U(IV) deposits begin to grow. In this phase, the deposits catalyze the reduction of U(V) to U(IV), resulting an increase of the reduction current. Finally, in the transformation phase, U(IV) hydroxide species transform into U dioxide having more stable state.
 and O
and O /H
/H O adsorption revealed by in situ XAFS and hard X-ray photoelectron spectroscopy
O adsorption revealed by in situ XAFS and hard X-ray photoelectron spectroscopyCui, Y.*; Harada, Yoshihisa*; Hatanaka, Tatsuya*; Nakamura, Naoki*; Ando, Masaki*; Yoshida, Toshihiko*; Ikenaga, Eiji*; Ishii, Kenji*; Matsumura, Daiju; Li, R.*; et al.
ECS Transactions, 72(8), p.131 - 136, 2016/10
Times Cited Count:1 Percentile:48.67(Electrochemistry) -ray irradiation
-ray irradiationMotooka, Takafumi; Komatsu, Atsushi; Tsukada, Takashi; Yamamoto, Masahiro
ECS Transactions, 53(21), p.25 - 32, 2013/05
Times Cited Count:3 Percentile:84.68(Electrochemistry)
Kato, Chiaki; Ueno, Fumiyoshi; Yamamoto, Masahiro; Ban, Yasutoshi; Uchiyama, Gunzo; Nojima, Yasuo*; Fujine, Sachio*
ECS Transactions, 53(21), p.45 - 55, 2013/05
Times Cited Count:2 Percentile:76.48(Electrochemistry)Neptunium ion contained as one of the fission products in reprocessing solutions is known as a corrosion accelerator of the stainless steel. But it is not clear why remarkable acceleration of corrosion is caused by a slight amount of the Np ion in boiling nitric acid solution. Neptunium has several oxidation states in nitric acid solution. These changeable oxidation states of Np in nitric acid solution are regarded as the cause. Therefore an evaluation of the electrochemical behaviors on stainless steel in nitric acid solution related to the oxidation state of Np is required in order to understand the corrosion acceleration mechanism. A specially designed electrochemical test cell integrated with optical cell for spectroscopic analysis was used for this purpose. From results of electrochemical tests, cathodic reaction on stainless steel was activated by Np ions. Np(VI) ion made the corrosion potential shift nobler than Np(V) and nobler corrosion potential causes increasing corrosion current and accelerating corrosion of stainless steel in nitric acid solution. Np(V) was easily oxidized to Np(VI) in nitric acid solution and Np(VI) was the stable state in boiling 3M-HNO . It was considered that role of Np ions was that of mediator to accelerate corrosion due to activating cathodic reaction and re-oxidizing cycle in boiling 3M-HNO
. It was considered that role of Np ions was that of mediator to accelerate corrosion due to activating cathodic reaction and re-oxidizing cycle in boiling 3M-HNO .
.
Oyaizu, Makoto; Isobe, Kanetsugu; Yamanishi, Toshihiko
ECS Transactions, 50(50), p.63 - 69, 2013/00
Times Cited Count:3 Percentile:80.7(Electrochemistry)The effects of tritiated water on the passivation behavior of SUS304 stainless steel were electrochemically studied by anodic polarization measurements and diachronic measurements of open circuit potential with changing tritium concentration and dissolved oxygen concentration as parameters in the electrolyte of 1N sulfuric acid solution, where the passivation inhibitory effects by tritiated water could be clearly observed. As a result, it was found that the passivation would be proceed with two steps. The effects of tritiated water could be observed in both of two steps; delay in the first step and deceleration in the second step. From these results, it was suggested that the passivation inhibitory effect might be promoted by further oxidation and sequential dissolution of Cr by radiolysis products.
by radiolysis products.
Yoshimura, Kimio; Koshikawa, Hiroshi; Yamaki, Tetsuya; Maekawa, Yasunari; Yamamoto, Kazuya*; Shishitani, Hideyuki*; Asazawa, Koichiro*; Yamaguchi, Susumu*; Tanaka, Hirohisa*
ECS Transactions, 50(2), p.2075 - 2081, 2012/10
no abstracts in English
Yoshida, Miru*; Zhao, Y.; Fujita, Masahiro*; Ohira, Akihiro*; Takeoka, Yuko*; Koizumi, Satoshi*; Rikukawa, Masahiro*
ECS Transactions, 50(2), p.1045 - 1053, 2012/10
Sawada, Shinichi; Maekawa, Yasunari
ECS Transactions, 41(1), p.2125 - 2133, 2011/10
Times Cited Count:6 Percentile:79.85(Electrochemistry)In order to improve the performance of the radiation-grafted polymer electrolyte membranes (PEMs), it is important to characterize the PEMs prepared by various conditions. In this study, we investigated the proton conduction properties of grafted PEMs based on crosslinked poly(tetrafluoroethylene) (cPTFE) (perfluoropolymer) and poly(etheretherketone) (PEEK) (hydrocarbon polymer). For obtaining a deep insight, the self-diffusion coefficient of protons, D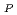 , was calculated. In the case of PEEK PEMs, the D
, was calculated. In the case of PEEK PEMs, the D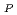 did not depend on the ion exchange capacity (IEC). On the other hand, for cPTFE PEMs, the D
did not depend on the ion exchange capacity (IEC). On the other hand, for cPTFE PEMs, the D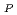 prominently increased with the IEC. The small D
prominently increased with the IEC. The small D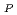 at low IEC can be related to the high crystallinity of the base cPTFE film. Due to PTFE crystallites, poly(styrene sulfonic acid) graft chains were not homogeneously introduced. As a result, the ion-channnel network structures should not be well-connected, thereby restricting the proton mobility.
at low IEC can be related to the high crystallinity of the base cPTFE film. Due to PTFE crystallites, poly(styrene sulfonic acid) graft chains were not homogeneously introduced. As a result, the ion-channnel network structures should not be well-connected, thereby restricting the proton mobility.
Yamaki, Tetsuya; Nuryanthi, N.*; Koshikawa, Hiroshi; Asano, Masaharu; Sawada, Shinichi; Hasegawa, Shin; Maekawa, Yasunari; Voss, K.-O.*; Trautmann, C.*; Neumann, R.*
ECS Transactions, 35(24), p.1 - 12, 2011/05
Times Cited Count:7 Percentile:93.89(Electrochemistry)Our focus has been placed on ion track membranes of poly(vinylidene fluoride) (PVDF), a type of fluoropolymer, because of their superior chemical, mechanical and ferro-electric properties. The aim of this study is to investigate the formation of the PVDF track membranes in more detail by electrolytic conductometry. Interestingly, application of a higher voltage to the conductometry cell as well as irradiation with a higher-LET beam promoted track etching up to breakthrough probably because electrophoretic migration of dissolved products occurred out of each pore.
 gate dielectrics for SiC power devices
gate dielectrics for SiC power devicesWatanabe, Heiji*; Kirino, Takashi*; Uenishi, Yusuke*; Chanthaphan, A.*; Yoshigoe, Akitaka; Teraoka, Yuden; Mitani, Shuhei*; Nakano, Yuki*; Nakamura, Takashi*; Hosoi, Takuji*; et al.
ECS Transactions, 35(2), p.265 - 274, 2011/05
Times Cited Count:8 Percentile:93.22(Electrochemistry)Watanabe, Heiji*; Hosoi, Takuji*; Kirino, Takashi*; Uenishi, Yusuke*; Chanthaphan, A.*; Ikeguchi, Daisuke*; Yoshigoe, Akitaka; Teraoka, Yuden; Mitani, Shuhei*; Nakano, Yuki*; et al.
ECS Transactions, 41(3), p.77 - 90, 2011/00
Times Cited Count:5 Percentile:90.72(Electrochemistry)Sawada, Shinichi; Yamaki, Tetsuya; Ozawa, Taku*; Suzuki, Akihiro*; Terai, Takayuki*; Maekawa, Yasunari
ECS Transactions, 33(1), p.1067 - 1078, 2010/10
Times Cited Count:7 Percentile:89.19(Electrochemistry)In order to investigate water transport in polymer electrolyte membranes (PEMs), we calculated the self-diffusion coefficient of water, D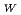 , by dissipative particle dynamics (DPD) simulation. The simulation target materials are Nafion and radiation-grafted PEMs in the fully-hydrated states. D
, by dissipative particle dynamics (DPD) simulation. The simulation target materials are Nafion and radiation-grafted PEMs in the fully-hydrated states. D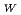 was obtained by the following steps: (1) molecular modeling with the coarse-grained particles representing groups of several atoms; (2) calculation of the water particle diffusivity, D
was obtained by the following steps: (1) molecular modeling with the coarse-grained particles representing groups of several atoms; (2) calculation of the water particle diffusivity, D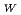
 , in the PEMs; (3) determination of the unit time in the DPD simulation; (4) conversion of D
, in the PEMs; (3) determination of the unit time in the DPD simulation; (4) conversion of D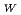
 of the PEMs into D
of the PEMs into D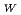 in the standard SI unit. Interestingly, D
in the standard SI unit. Interestingly, D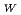 was found to decrease with the diffusion time period,
was found to decrease with the diffusion time period,  t, probably owing to the geometrical confinement effect by water-transport hydrophilic regions. Quantitative analysis of this D
t, probably owing to the geometrical confinement effect by water-transport hydrophilic regions. Quantitative analysis of this D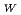 -
- t relationship provided us with the size of hydrophilic regions.
t relationship provided us with the size of hydrophilic regions.
Asazawa, Koichiro*; Yamamoto, Kazuya*; Yamada, Koji*; Tanaka, Hirohisa*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo; Atanassov, P.*
ECS Transactions, 33(1), p.1751 - 1755, 2010/10
CoPPyC were analyzed with X-ray adsorption fine structure (XAFS) measurements. Acid-treated CoPPyC (CoPPyC-AT) has higher activity than PPyC for oxygen reduction reaction. From the analysis of EXAFS of Co, CoPPyC electrocatalysts as synthesized consist of two peaks. The peak around 1.6  was assigned to Co-N and/or Co-O shells. The second peak around 2.6
was assigned to Co-N and/or Co-O shells. The second peak around 2.6  was assigned to Co-O-Co shells originated from cobalt hydroxide. CoPPyC-AT showed only one peak of assigned to Co-N and/or Co-O, and it indicates that cobalt hydroxide is removed by acid treatment. It is clear that a coexistence of cobalt and nitrogen in CoPPyC-AT shows specific performance, and pyrolysis is not necessary to make correlation of Co-N.
was assigned to Co-O-Co shells originated from cobalt hydroxide. CoPPyC-AT showed only one peak of assigned to Co-N and/or Co-O, and it indicates that cobalt hydroxide is removed by acid treatment. It is clear that a coexistence of cobalt and nitrogen in CoPPyC-AT shows specific performance, and pyrolysis is not necessary to make correlation of Co-N.
Yamamoto, Masahiro; Motooka, Takafumi; Ueno, Fumiyoshi; Kato, Chiaki
ECS Transactions, 25(40), p.23 - 30, 2010/00
Times Cited Count:1 Percentile:35.77(Electrochemistry)Corrosion rates of stainless steels have been accelerated by existence of oxidizing ions, i.e. Cr(VI) and V(V). However, the mechanism of acceleration effect by these ions was not clear. The results of examination for the effect showed that the acceleration effect of Cr was larger than that of V. But, the effect was rapidly decreased because of the reduction from Cr(VI) to Cr(III). Although, the acceleration effect of V stayed constant. And V(IV) was more rapidly re-oxidized to V(V) than Cr(VI) in boiling nitric acid solution. So, the corrosion rate in the solution containing V ion does not change with time because of high re-oxidation rate of V ion.
Yamaki, Tetsuya; Sawada, Shinichi; Asano, Masaharu; Maekawa, Yasunari; Yoshida, Masaru*; Gubler, L.*; Alkan G rsel, S.*; Scherer, G. G.*
rsel, S.*; Scherer, G. G.*
ECS Transactions, 25(1), p.1439 - 1450, 2009/10
Times Cited Count:4 Percentile:83.35(Electrochemistry)A multiply-crosslinked polymer electrolyte membrane was prepared by the radiation-induced co-grafting of styrene and a bis(vinyl phenyl)ethane (BVPE) crosslinker into a radiation-crosslinked polytetrafluoroethylene (cPTFE) film. We then investigated its hydrogen/oxygen fuel-cell performance at 60 and 80 C in terms of the effect of radiation and chemical crosslinking. At 60
C in terms of the effect of radiation and chemical crosslinking. At 60 C, all the membranes initially exhibited similar performance, but only the cPTFE-based membranes were durable at 80
C, all the membranes initially exhibited similar performance, but only the cPTFE-based membranes were durable at 80 C, indicating the necessity of radiation crosslinking in the PTFE main chains. Importantly, cell performance of the multiply-crosslinked membrane was found high enough to reach that of a Nafion112 membrane. This is probably because the BVPE crosslinks in the graft component improved the membrane-electrode interface in addition to membrane durability. After severe OCV hold tests at 80 and 95
C, indicating the necessity of radiation crosslinking in the PTFE main chains. Importantly, cell performance of the multiply-crosslinked membrane was found high enough to reach that of a Nafion112 membrane. This is probably because the BVPE crosslinks in the graft component improved the membrane-electrode interface in addition to membrane durability. After severe OCV hold tests at 80 and 95 C, the performance deteriorated, while no significant change was observed in ohmic resistivity. Accordingly, our membranes seemed so chemically stable that an influence on overall performance loss could be negligible.
C, the performance deteriorated, while no significant change was observed in ohmic resistivity. Accordingly, our membranes seemed so chemically stable that an influence on overall performance loss could be negligible.
 measurements of oxide film electric resistance in high temperature water
measurements of oxide film electric resistance in high temperature waterUchida, Shunsuke; Sato, Tomonori; Kakinuma, Nagao*; Miyazawa, Takahiro*; Sato, Yoshiyuki*; M kel
kel , K.*
, K.*
ECS Transactions, 2(25), p.39 - 50, 2007/00
no abstracts in English
Fujieda, Shinji*; Terai, Masayuki*; Saito, Motofumi*; Toda, Akio*; Miura, Yoshinao*; Liu, Z.*; Teraoka, Yuden; Yoshigoe, Akitaka; Wilde, M.*; Fukutani, Katsuyuki*
ECS Transactions, 6(3), p.185 - 202, 2007/00
In order to find how bias temperature instability occurs in advanced gate stacks, we will review experimental results of our investigation on SiO , plasma-nitrided SiON, HfSiON and HfSiON with Ni-silicide electrodes. It thus seems that we need to clarify and control the chemical and physical influences on the insulator bulk and the insulator/Si interface caused by newly incorporated materials and process technologies, in order to ensure the reliability of bias temperature instability for advanced gate stacks.
, plasma-nitrided SiON, HfSiON and HfSiON with Ni-silicide electrodes. It thus seems that we need to clarify and control the chemical and physical influences on the insulator bulk and the insulator/Si interface caused by newly incorporated materials and process technologies, in order to ensure the reliability of bias temperature instability for advanced gate stacks.
 Si/SiGe]
Si/SiGe] (n = 1-2) multi-layered structures for spintronics application
(n = 1-2) multi-layered structures for spintronics applicationSado, Taizo*; Ueda, Koji*; Ando, Yuichiro*; Kumano, Mamoru*; Narumi, Kazumasa; Maeda, Yoshihito; Miyao, Masanobu*
ECS Transactions, 11(6), p.473 - 479, 2007/00
Our recent progresses in epitaxial growth of Fe Si on Ge substrates are reviewed. Single crystalline Fe
Si on Ge substrates are reviewed. Single crystalline Fe Si layers with atomically flat interfaces were achieved on Ge(111) substrates by optimizing growth conditions at low temperatures (60
Si layers with atomically flat interfaces were achieved on Ge(111) substrates by optimizing growth conditions at low temperatures (60 200
200  C). Thermal stability of it was guaranteed up to 400
C). Thermal stability of it was guaranteed up to 400  C. In addition, epitaxial growth of mixed layers composed of Fe
C. In addition, epitaxial growth of mixed layers composed of Fe Si, FeGe, and FeSi on Ge substrates at 400
Si, FeGe, and FeSi on Ge substrates at 400  C is reported. Finally, epitaxial growth of Fe
C is reported. Finally, epitaxial growth of Fe Si/Ge/Fe
Si/Ge/Fe Si/Ge structures is discussed. These results will be a powerful tool to open up SiGe related spintronics.
Si/Ge structures is discussed. These results will be a powerful tool to open up SiGe related spintronics.
 Si on Ge substrate
Si on Ge substrateKumano, Mamoru*; Ando, Yuichiro*; Ueda, Koji*; Sado, Taizo*; Narumi, Kazumasa; Maeda, Yoshihito; Miyao, Masanobu*
ECS Transactions, 11(6), p.481 - 485, 2007/00
The effects of the Fe/Si ratios on molecular beam epitaxy (MBE) of Fe Si on Ge substrate have been investigated in a wide range of growth temperatures (60
Si on Ge substrate have been investigated in a wide range of growth temperatures (60 300
300  C). From XRD measurements, it was found that Fe
C). From XRD measurements, it was found that Fe Si layers were epitaxially grown on Ge(111) substrates at 60
Si layers were epitaxially grown on Ge(111) substrates at 60 200
200  C under the stoichiometric (Fe:Si = 3:1) and non-stoichiometric (Fe:Si = 4:1) conditions. From RBS measurement, it was found that atomic mixing of Fe and Ge at Fe
C under the stoichiometric (Fe:Si = 3:1) and non-stoichiometric (Fe:Si = 4:1) conditions. From RBS measurement, it was found that atomic mixing of Fe and Ge at Fe Si/Ge interfaces began at a growth temperature of 300
Si/Ge interfaces began at a growth temperature of 300  C. In the case of MBE under the stoichiometric condition, the crystallinity of Fe
C. In the case of MBE under the stoichiometric condition, the crystallinity of Fe Si is significantly improved compared to the non-stoichiometric condition. As a result, very low
Si is significantly improved compared to the non-stoichiometric condition. As a result, very low 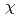
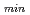 was obtained in a wide temperature (60
was obtained in a wide temperature (60 200
200  C) under the stoichiometric condition. From the transmission electron microscopy measurements, it was shown that high-quality DO3-type Fe
C) under the stoichiometric condition. From the transmission electron microscopy measurements, it was shown that high-quality DO3-type Fe Si/Ge structures with atomically flat interfaces were realized at a low temperature (
Si/Ge structures with atomically flat interfaces were realized at a low temperature ( 200
200  C) under the stoichiometric condition.
C) under the stoichiometric condition.
 2 surface by photoemission spectroscopy
2 surface by photoemission spectroscopySuemitsu, Maki*; Kato, Atsushi*; Togashi, Hideaki*; Konno, Atsushi*; Yamamoto, Yoshihisa*; Teraoka, Yuden; Yoshigoe, Akitaka; Enta, Yoshiharu*; Narita, Yuzuru*
ECS Transactions, 3(2), p.311 - 316, 2006/00
Initial thermal oxidation of Si(110) surface has been investigated by using real-time X-ray photoemission spectroscopy with synchrotron radiation. The Si(110) initial oxidation is characterized by presence of a rapid oxidation just after the introduction of gaseous oxygen molecules. Peak separation of the O1s photoemission spectra suggests the presence of at least two distinct oxidation sites on the surface, which may reflect the complicated surface structure of the Si(110)-16 2 reconstruction.
2 reconstruction.
Yamaki, Tetsuya; Kozone, Yuichi; Hiroki, Akihiro; Asano, Masaharu; Kubota, Hitoshi*; Yoshida, Masaru
ECS Transactions, 3(1), p.103 - 112, 2006/00
Fluoropolymer-based electrolyte membranes for water electrolyzers and fuel cells were prepared by using heavy ion beams from the cyclotron accelerator. The preparation method for these so-called "nano-scale structure-controlled membranes" involves (1) the swift heavy ion irradiation of polyvinylidene fluoride films and subsequent chemical etching to obtain cylindrical pores with a diameter of 100 nm, and (2) the filling of proton-conducting polymer chains into the etched pores by  -ray-induced graft polymerization. The proton transport only in the thickness direction was observed for the resulting membranes with controlled ion exchange capacities, indicating the formation of one-dimensional straight proton-conducting pathways parallel to the ion-beam incident axis. The membranes exhibited a lower water uptake and reduced methanol permeability compared to commercially-available Nafion probably due to the restricted structures.
-ray-induced graft polymerization. The proton transport only in the thickness direction was observed for the resulting membranes with controlled ion exchange capacities, indicating the formation of one-dimensional straight proton-conducting pathways parallel to the ion-beam incident axis. The membranes exhibited a lower water uptake and reduced methanol permeability compared to commercially-available Nafion probably due to the restricted structures.